Sorry! This page under construction!
Enzyme Kinetics
Basic Definitions
[ERM Main Menu]
[About the ERM]
[Cleland notation]
[Enzyme kinetics]
[Alternate nomenclature]
[Publication]
Catalyst
It is a substance that increases the reaction rate without modifying the overall standard
Gibbs-energy change in the reaction. This reaction called catalysed reaction.
Enzyme
It is a protein that acts as a catalyst and a regulator of biochemical processes.
Enzymes increase the rate of reactions without themselves being altered in the process of
substrate conversion to product.
Usually free form of enzyme is designated by E.
Substrate/Product
Any organic or inorganic substance being the reactant in a catalysed reaction.
Substrates are designated by the letters A, B, C, D,..., in the
order in which they bind to the enzyme.
Products are designated by P, Q, R, S,..., in the order in which they
release from the enzyme.
Inhibitor
An organic or inorganic substance that lowers the catalytic function of an enzyme.
The inhibitor is allosteric if it binds an allosteric site. An inhibitor can decrease the
apparent catalytic constant of the enzyme by many different mechanisms.
Inhibitors are indicated as I.
Reaction rate
Rates of reactions are measured by change in reactant amounts (concentration) with
time (T or t).
Very simple representation of change of reactant A (substrate) to the
reactant P (product) is
A |
 |
P |
The rate can be expressed as either the decrease in concentration of A or the
increase in concentration of P. Negative sign indicates decrease of reactant.
Ways to express a rate for this reaction:
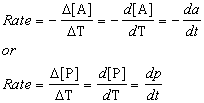
Rate is equal reaction velocity and indicates as v, i.e.:
v = - dA/dt = dP/dt
Elementary reaction, rate constants
The overall enzyme catalysed reaction involving a single substrate and a single product
was visualized as
 The double arrows indicate that the reaction occurs in both directions.
The double arrows indicate that the reaction occurs in both directions.
The reaction in which no reaction intermediates have been detected
is called an elementary reactions or a single step.
It is represented as:
| A + E |

k1 |
EA |
| EA |
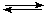 |
EP |
| EP |

k2 |
P + E, |
where EA, EP - rapid exchange comlexes;
k1, k2 called rate constants.
Steady-State Approximation
....
....
Nomenclature of Reaction Mechanisms
We represent a mechanism of enzymatic catalysis using a modified version of the Cleland
nomenclature (W. W. Cleland, Adv. Enzymol., 1967, v.29, pp.1-32.).
The nomenclature and notation described in this work have proven very useful in discussing
various mechanisms and in teaching enzyme kinetics to graduate students. The number of defined
kinetic constants is not large and these correspond either to maximum velocities,
Michaelis constants, product inhibition constants, or equilibrium constants for steps in
the mechanism.
Kinetic Parameters
Maximal Velocity
Maximum, or rate limiting, velocity is the most important parameter.
It is a theoretical quantity giving the asymptotic value of the reaction
rate provided all reactants of the reaction are fully saturating the catalytic
(active) center and all inhibitory effects of reactants upon the enzyme are
experimentally or theoretically excluded.
Maximal velocities in forward and reverse directions are designated as
V1 and V2 and are represent velocities with all
substrates saturating (at infinite concentration) and all products at zero concentration.
Total Enzyme Concentration
Total enzyme concentration is the sum of all transitory complex concentrations and
is indicated as Et.
Michaelis Constant
In the simplest case, defines the substrate concentration at which the enzyme activity exhibits
half the maximum velocity. The Michaelis constant can be interpreted in
a number of ways, but it always characterizes the enzyme-substrate interactions,
not the enzyme itself. In certain cases, Michaelis constant nearly equals to the
dissociation constant for the enzyme-substrate complex and can thus serves
a measure of the affinity of an enzyme for its substrate.
In a multisubstrate reaction, characterizes an enzyme affinity with respect to one
of the reactants. If concentrations of other reactants are fixed the apparent Michaelis
constant gives the substrate concentration at which the reaction rate attains 50% of its
maximum value. By contrast to the true Michaelis constant of a unireactant reaction,
the apparent Michaelis constant is by no means a 'constant' but a function of concentrations
of other reactants.
Michaelis constants are limiting constants in that they equal the concentration
of the particular substrate that yields half-maximal velocity when all the other substrates
in the given direction are saturating. The Michaelis constant for a given substrate
at unsaturating cosubstrate concentrations can be greater then, less then, or equal to
the limiting value, depending on the kinetic mechanism.
We used Modified Cleland nomenclature and our Michaelis constants are indicated as
Ka, Kp, and so on.
Inhibition Constant
Where addition of a reactant gives a non-central transitory complex,
the inhibition constant for this reactant is also the dissociation constant for this complex.
The inhibition constants are indicated as Kia, Kip, and so on...
Equilibrium Constant
It is indicated as Keq.
Isoinhibition Constant
They are indicated as Kiia, Kiip, and so on.
[ERM Main Menu]
[About the ERM]
[Cleland notation]
[Enzyme kinetics]
[Alternate nomenclature]
[Publication]
Russian Academy of Science
Institute of Theoretical and Experimental Biophysics
Laboratory of Metabolic Simulation and Bioinformatics
Pushchino, Moscow reg., Russia, 142290.
Created by Milya Galimova. Last updated October 18, 2002.